Abstract
Introduction: Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment paradigm for non-Hodgkin's lymphoma. However, most CAR T-cell trials excluded primary central nervous system lymphoma (PCNSL) patients due to the neurotoxicity concern. This systematic review and meta-analysis aimed to explore the efficacy and safety of intravenous CAR T-cell therapy in PCNSL patients.
Methods: We searched Pubmed, Scopus, Cochrane Library, and Embase for relevant articles from inception to June 26, 2022. We also hand-searched conference abstracts. The inclusion criteria for a study were: i) all participants being at least 16 years old; ii) at least 3 participants having PCNSL at the time of intravenous CAR T-cell infusion; and iii) reporting the rates of best complete response (BCR), best objective response (BOR), cytokine release syndrome (CRS), or Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). We performed single-arm meta-analyses to quantitatively synthesize the outcomes using cumulative event rates (CERs) and 95% confidence intervals (95% CIs).
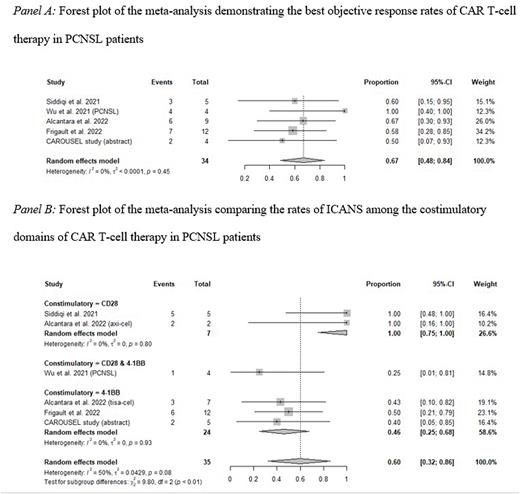
Results: This review included 5 studies involving 35 participants. Intravenous CAR T-cell therapies included axicabtagene ciloleucel, tisagenlecleucel, obecabtagene autoleucel, lab manufactured anti-CD19, and sequential anti-CD19/22 CAR T-cell. The BCR and BOR were 56.0% (95% CI: 37.3-74.1%) and 66.9% (95% CI: 48.3-83.6%), respectively. The response rates were not significantly different among the costimulatory domains, including CD28, 4-1BB, and a combination of CD28 and 4-1BB. The rates of CRS, grade ≥ 3 CRS, ICANS, and grade ≥ 3 ICANS were 74.2% (95% CI: 55.7-89.6%), 0.6% (95% CI: 0-9.8%), 57.5% (95% CI: 31.3-82.0%), and 18.3% (95% CI: 5.4-35.0%). Subgroup analysis demonstrated that ICANS occurred more frequently in CAR T-cells with CD28 costimulatory domain (100%, 95% CI: 75.0-100%) than CAR T-cells with 4-1BB costimulatory domain (45.8% 95% CI: 24.8-67.5%). However, sICANS rates among costimulatory domains were not significantly different.
Conclusion: The efficacy and safety profiles of intravenous CAR T-cell therapies in PCNSL patients are efficient and acceptable toxicity. Further studies should explore the effect of costimulatory domains on the side effects of CAR T-cell therapy.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal